Some Questions I Had About Pharma and Drug Development
Questions around R&D spending
- Can I neatly split up expected costs per trial period: preclinical, clinical? Or
does it vary too much?
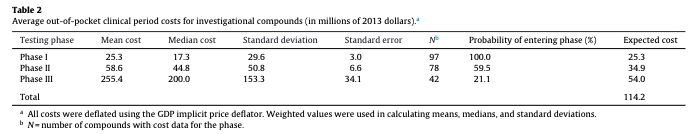
- This is Figure 3 in Entrepreneur's Guide to a Biotech Startup by Kolchinsky which was written in 2001:
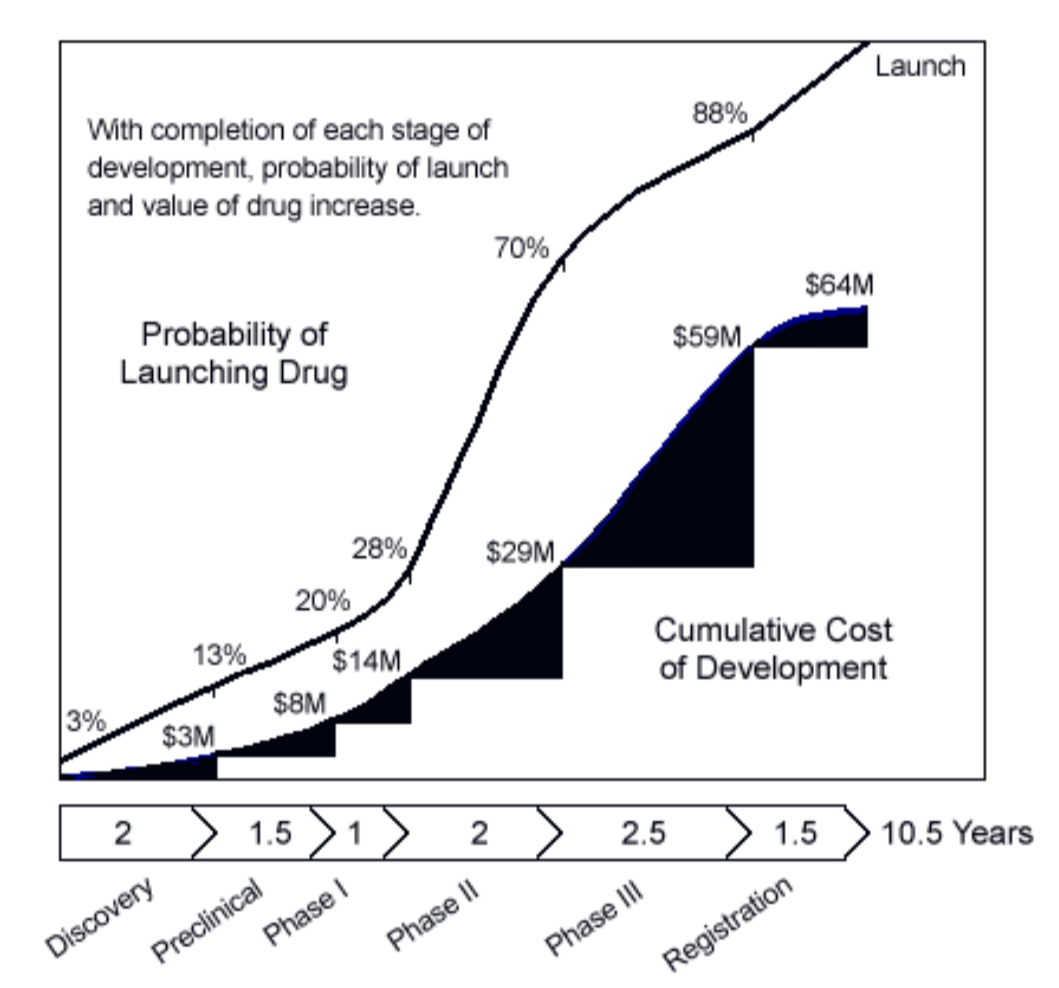
- Have things changed since 2001? Is this even accurate?
- But why does each trial cost what it does?
- What drug types are most expensive and why?
- this site
gives costs for different types of drugs. Average cost for clinical trial for
one patient is $36.5K (which seems crazy high!). For cancer, it's $60K. For
infectious diseases, it's $17K.
- The general cost breakdown is:
- 20% on "clinical procedures"
- 20% on "staff"
- 14% on "site monitoring"
- 12% on "site retention"
- 8% on "lab costs"
- What would a very cheap drug to develop look like?
- What class of drugs does a poorly-funded startup have a chance of developing?
- How cheaper does AlphaFold make things?
- How much does global pharma spend on R&D?
- Where is this allocated? How much to drug design? What other parts of R&D exist?
- Does it now make sense to get more granularity on where money is allocated? Like,
what types of drugs get the most funding to be developed and why? What get the
least?
- What areas have just a single big company or two looking at them? Where are
things likely to not be very competitive?
- What do more efficient clinical trials look like?
- "Adaptive designs in clinical trials: why use them, and how to run and report them"
- Another thing from just thinking about this is that it seems we're repeating an
insane amount of work for each thing? Suppose we know two drugs are pretty similar
for how they work; does the FDA allow for fewer trials then? Can we be more
Bayesian here? Can ML help with that?
- Instead of just being like "we're not being Bayesian" or "eh, the FDA is letting
us be pretty Bayesian", just look at the evidence! Look at the history of
drugs in clinical trials. How close were they to other drugs at the time? It
sure seems like there's just a few classes of drugs when I go to Walmart Pharmacy
for eg pain drugs.
- Where is R&D focusing?
- Are there branches of the FDA that are easier to get past?
Some ideas for things I should read
- Maybe the Genentech book, but I'm not so sure. That's more historical; I don't
care yet, right?
- The FDA's setup.
N Horwitz says you'll be surprised what's not in it?
- SEC to see company financials?
- https://medium.com/@david.eil/does-america-have-to-overpay-for-health-care-to-drive-innovation-8b974922d8c
- https://www.cbo.gov/publication/57126
- Read Entrepreneur's Guide to Biotech Startup
- Read Pharmagellan's Guide to Clinical Trials
- Part 2 Special Topics has a bunch of independent interesting things I could
Anki in 30 minutes a piece.
- Read How Drugs Work
- Read Which Country Has the World's Best Health Care by Emanuel
- https://slatestarcodex.com/2016/09/07/reverse-voxsplaining-brand-name-drugs/
- https://www.science.org/content/blog-post/drugs-2015-2021